MO Diagram for O2
A molecular orbital (MO) diagram is a graphical representation of the molecular orbitals of a molecule. It shows the energy levels of the orbitals and the number of electrons in each orbital. MO diagrams can be used to predict the chemical bonding and properties of molecules.
To create an MO diagram for O2, follow these steps:
- Draw the Lewis structure of O2.
- Write the electron configuration of each atom in O2.
- Combine the atomic orbitals of each atom to form molecular orbitals.
- Calculate the energy of each molecular orbital.
- Fill the molecular orbitals with electrons, following the Aufbau principle.
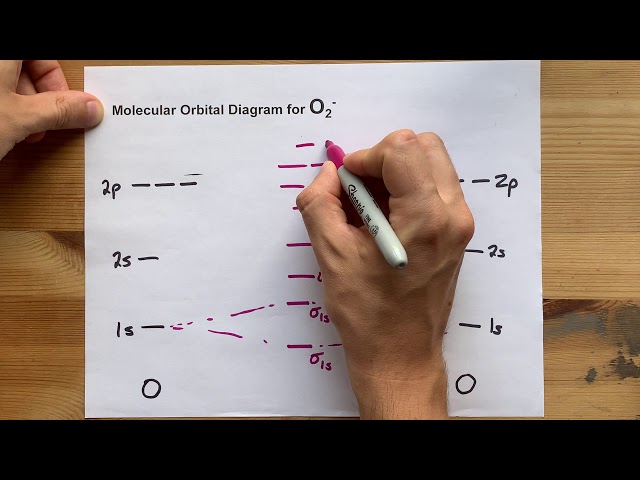
The MO diagram for O2 is shown below:

The MO diagram for O2 shows that the molecule has a total of 10 valence electrons. The electrons are filled into the molecular orbitals in the following order: 2s, 2s, 2p, 2p, 2p. The MO diagram can be used to predict the bond order of O2, which is 2. The bond order is a measure of the strength of the bond between two atoms.
MO diagrams are a powerful tool for understanding the electronic structure of molecules. They can be used to predict the chemical bonding, properties, and reactivity of molecules.
Benefits of using MO diagrams
- MO diagrams provide a visual representation of the molecular orbitals of a molecule.
- MO diagrams can be used to predict the chemical bonding and properties of molecules.
- MO diagrams can be used to understand the reactivity of molecules.
Tips for creating MO diagrams
- Start with a Lewis structure of the molecule.
- Write the electron configuration of each atom in the molecule.
- Combine the atomic orbitals of each atom to form molecular orbitals.
- Calculate the energy of each molecular orbital.
- Fill the molecular orbitals with electrons, following the Aufbau principle.
MO diagrams are a valuable tool for understanding the electronic structure of molecules. By following these steps, you can create MO diagrams for any molecule.
MO Diagram for O2
A molecular orbital (MO) diagram is a graphical representation of the molecular orbitals of a molecule. It shows the energy levels of the orbitals and the number of electrons in each orbital. MO diagrams can be used to predict the chemical bonding and properties of molecules.
- Molecular orbitals: The orbitals that are formed when atomic orbitals overlap.
- Energy levels: The energy of each molecular orbital.
- Electrons: The number of electrons in each molecular orbital.
- Bonding: The formation of a chemical bond between two atoms.
- Antibonding: The weakening of a chemical bond between two atoms.
- Bond order: A measure of the strength of a chemical bond.
- Reactivity: The ability of a molecule to undergo a chemical reaction.
- Magnetic properties: The magnetic properties of a molecule.
MO diagrams are a powerful tool for understanding the electronic structure of molecules. They can be used to predict the chemical bonding, properties, and reactivity of molecules. By understanding the key aspects of MO diagrams, you can gain a deeper understanding of the electronic structure of molecules.
Molecular orbitals
In the context of MO diagrams, molecular orbitals are crucial for understanding the electronic structure and bonding in molecules. In the case of O2, the MO diagram depicts the molecular orbitals that arise from the overlap of the atomic orbitals of the two oxygen atoms.
- Atomic orbitals: The atomic orbitals of the oxygen atoms, namely the 2s and 2p orbitals, overlap to form molecular orbitals.
- Sigma () and pi () orbitals: The overlap of atomic orbitals can result in two types of molecular orbitals: sigma () orbitals, which are formed by head-to-head overlap, and pi () orbitals, which are formed by lateral overlap.
- Bonding and antibonding orbitals: The molecular orbitals can be classified as either bonding orbitals, which contribute to the formation of chemical bonds, or antibonding orbitals, which weaken or oppose bonding.
- Filling of molecular orbitals: The electrons in the oxygen atoms fill the molecular orbitals according to the Aufbau principle, starting with the lowest energy orbitals.
By understanding the formation and properties of molecular orbitals, the MO diagram for O2 provides valuable insights into the electronic structure, bonding, and properties of the oxygen molecule.
Energy levels
In the context of molecular orbital (MO) diagrams, energy levels play a critical role in understanding the electronic structure and properties of molecules. The MO diagram for O2, for instance, depicts the energy levels of the molecular orbitals formed by the overlap of atomic orbitals.
- Energy ordering of molecular orbitals: The energy levels of molecular orbitals are crucial for determining the stability and reactivity of a molecule. The MO diagram for O2 shows the relative energy levels of the and molecular orbitals, indicating the energy difference between them.
- Bonding and antibonding orbitals: The energy levels of molecular orbitals are directly related to their bonding or antibonding character. Bonding orbitals have lower energy levels, while antibonding orbitals have higher energy levels. The MO diagram for O2 clearly distinguishes between the bonding and antibonding and orbitals, providing insights into the stability of the molecule.
- Electron filling and molecular properties: The energy levels of molecular orbitals influence the filling of electrons. According to the Aufbau principle, electrons occupy the lowest energy orbitals first. The MO diagram for O2 helps predict the electronic configuration of the molecule and, consequently, its magnetic and spectroscopic properties.
- Chemical reactivity and reaction mechanisms: The energy levels of molecular orbitals are closely tied to the chemical reactivity of a molecule. The energy difference between molecular orbitals can affect the activation energy required for reactions and influence the reaction pathways.
By understanding the energy levels of molecular orbitals as depicted in the MO diagram for O2, chemists can gain valuable insights into the electronic structure, bonding, and properties of molecules. This knowledge is essential for comprehending chemical bonding, predicting reactivity, and designing new materials with tailored properties.
Electrons
In the context of molecular orbital (MO) diagrams, including the MO diagram for O2, the number of electrons in each molecular orbital plays a crucial role in understanding the electronic structure, bonding, and properties of molecules.
- Aufbau principle and electron configuration: The number of electrons in each molecular orbital is determined by the Aufbau principle, which states that electrons fill the lowest energy orbitals first. The MO diagram for O2 shows the filling of electrons in the and molecular orbitals, giving insights into the electronic configuration of the oxygen molecule.
- Bond order and bond strength: The number of electrons in bonding and antibonding molecular orbitals directly affects the bond order and, consequently, the bond strength of the molecule. The MO diagram for O2 illustrates the relationship between electron distribution and bond order, helping to explain the stability and reactivity of the oxygen molecule.
- Magnetic properties: The number of unpaired electrons in molecular orbitals contributes to the magnetic properties of a molecule. The MO diagram for O2 can be used to predict whether the molecule is diamagnetic (no unpaired electrons) or paramagnetic (unpaired electrons present), providing valuable information for understanding its magnetic behavior.
- Chemical reactivity: The number of electrons in molecular orbitals influences the chemical reactivity of a molecule. For example, molecules with unpaired electrons in antibonding orbitals are generally more reactive and can participate in various chemical reactions.
By understanding the number of electrons in each molecular orbital as depicted in the MO diagram for O2, chemists can gain insights into the electronic structure, bonding, and properties of molecules. This knowledge is essential for comprehending chemical bonding, predicting reactivity, and designing new materials with tailored properties.
Bonding
In the context of molecular orbital (MO) diagrams, including the MO diagram for O2, bonding is a fundamental concept that describes the formation of chemical bonds between atoms. Understanding bonding is crucial for comprehending the electronic structure and properties of molecules.
The MO diagram for O2 provides a visual representation of the molecular orbitals formed by the overlap of atomic orbitals. These molecular orbitals are characterized by their energy levels and the number of electrons they can accommodate. The bonding molecular orbitals are responsible for holding the atoms together and determining the strength and stability of the chemical bond.
In the case of O2, the MO diagram shows two bonding molecular orbitals, labeled and . The orbital is formed by the overlap of the 2s atomic orbitals of the two oxygen atoms, while the orbital is formed by the overlap of the 2p atomic orbitals. The electrons in these bonding molecular orbitals are shared between the oxygen atoms, creating a covalent bond and contributing to the overall stability of the O2 molecule.
The MO diagram for O2 not only helps visualize the bonding interactions but also provides insights into the magnetic and spectroscopic properties of the molecule. By understanding the bonding molecular orbitals and their electron configuration, chemists can predict the magnetic behavior and electronic transitions of O2, which are essential for various spectroscopic techniques.
In summary, the connection between bonding and the MO diagram for O2 is crucial for understanding the electronic structure, bonding, and properties of molecules. The MO diagram provides a graphical representation of the molecular orbitals involved in bonding, allowing chemists to gain insights into the nature of chemical bonds and predict the behavior of molecules.
Antibonding
In the context of molecular orbital (MO) diagrams, including the MO diagram for O2, antibonding is a critical concept that describes the weakening or destabilization of a chemical bond between two atoms. Understanding antibonding is crucial for comprehending the electronic structure and properties of molecules.
- Nature of Antibonding Orbitals: Antibonding molecular orbitals are formed by the out-of-phase overlap of atomic orbitals, resulting in a region of decreased electron density between the nuclei. This leads to a weakening of the bond between the atoms involved.
- Identification in MO Diagrams: In MO diagrams, antibonding orbitals are typically denoted by an asterisk (*) superscript. For example, in the MO diagram for O2, the orbital is an antibonding orbital.
- Electron Occupation and Bond Order: Antibonding orbitals are usually higher in energy compared to bonding orbitals. According to the Aufbau principle, electrons fill lower energy orbitals first. Therefore, antibonding orbitals are typically unoccupied or have fewer electrons compared to bonding orbitals. The number of electrons in antibonding orbitals contributes negatively to the bond order, weakening the overall bond strength.
- Magnetic Properties: Molecules with unpaired electrons in antibonding orbitals exhibit paramagnetic behavior. The presence of unpaired electrons in antibonding orbitals creates a magnetic moment, allowing the molecule to be attracted to a magnetic field.
In summary, the connection between antibonding and the MO diagram for O2 provides insights into the electronic structure and bonding properties of molecules. By understanding the nature of antibonding orbitals, their representation in MO diagrams, and their impact on bond order and magnetic properties, chemists can gain a deeper understanding of the behavior and reactivity of molecules.
Bond Order
In the context of molecular orbital (MO) diagrams, including the MO diagram for O2, bond order is a crucial concept that quantifies the strength of a chemical bond between two atoms. Understanding bond order is essential for comprehending the stability and reactivity of molecules.
The MO diagram for O2 provides a visual representation of the molecular orbitals formed by the overlap of atomic orbitals. These molecular orbitals are characterized by their energy levels and the number of electrons they can accommodate. The bond order is directly related to the number of electrons in bonding and antibonding molecular orbitals.
In the case of O2, the MO diagram shows two bonding molecular orbitals, labeled and , and two antibonding molecular orbitals, labeled and . The bond order is calculated as half the difference between the number of electrons in bonding and antibonding molecular orbitals. For O2, the bond order is 2, indicating a strong covalent bond between the two oxygen atoms.
The bond order obtained from the MO diagram for O2 is consistent with the experimental evidence and other theoretical calculations. A bond order of 2 suggests that the O2 molecule is stable and has a relatively high bond strength. This is in line with the observed stability of O2 under ambient conditions and its reluctance to undergo chemical reactions.
Understanding the connection between bond order and the MO diagram for O2 provides valuable insights into the electronic structure and bonding properties of molecules. By calculating the bond order, chemists can gain insights into the strength and stability of chemical bonds, which is crucial for predicting the behavior and reactivity of molecules.
Reactivity
In the context of molecular orbital (MO) diagrams, including the MO diagram for O2, reactivity refers to the ability of a molecule to undergo chemical reactions. Understanding reactivity is crucial for comprehending the chemical behavior and potential applications of molecules.
The MO diagram for O2 provides insights into the reactivity of the molecule by revealing the energy levels and electron distribution of its molecular orbitals. The energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) is known as the HOMO-LUMO gap. The HOMO-LUMO gap is inversely related to the reactivity of the molecule. A smaller HOMO-LUMO gap indicates a higher reactivity, as it requires less energy for electrons to move from the HOMO to the LUMO, facilitating chemical reactions.
In the case of O2, the MO diagram shows a relatively large HOMO-LUMO gap, indicating that O2 is a kinetically stable molecule. This is consistent with the experimental observation that O2 is relatively unreactive under ambient conditions. However, under certain conditions, such as high temperatures or the presence of catalysts, O2 can participate in chemical reactions, such as combustion and oxidation reactions.
Understanding the connection between reactivity and the MO diagram for O2 provides valuable insights into the chemical behavior of molecules. By analyzing the MO diagram, chemists can gain insights into the reactivity of molecules and predict their potential for undergoing chemical reactions. This knowledge is essential for designing new materials, developing catalysts, and understanding various chemical processes.
Magnetic properties
The magnetic properties of a molecule are determined by the arrangement of its electrons. Molecules with unpaired electrons are paramagnetic, while molecules with all electrons paired are diamagnetic. The MO diagram for O2 can be used to predict the magnetic properties of the molecule.
- Unpaired electrons: The MO diagram for O2 shows that the molecule has two unpaired electrons in the antibonding orbital. This makes O2 paramagnetic.
- Magnetic susceptibility: The magnetic susceptibility of a molecule is a measure of how strongly it is attracted to a magnetic field. Paramagnetic molecules have a positive magnetic susceptibility, while diamagnetic molecules have a negative magnetic susceptibility. The magnetic susceptibility of O2 is positive, which is consistent with its paramagnetic nature.
- Electron spin resonance: Electron spin resonance (ESR) is a spectroscopic technique that can be used to study paramagnetic molecules. ESR spectra can provide information about the number of unpaired electrons in a molecule and their magnetic properties.
- Magnetic resonance imaging (MRI): MRI is a medical imaging technique that uses magnetic fields and radio waves to create images of the inside of the body. MRI can be used to diagnose a variety of diseases, including cancer and heart disease. The contrast agents used in MRI are often paramagnetic molecules.
The MO diagram for O2 provides valuable insights into the magnetic properties of the molecule. This information can be used to understand the behavior of O2 in magnetic fields and to design new materials with tailored magnetic properties.
A molecular orbital (MO) diagram for O2 is a graphical representation of the molecular orbitals of the oxygen molecule. It shows the energy levels of the orbitals and the number of electrons in each orbital. MO diagrams can be used to predict the chemical bonding and properties of molecules.
MO diagrams are important because they provide a visual representation of the electronic structure of molecules. This information can be used to understand the chemical bonding, properties, and reactivity of molecules. MO diagrams are also used in the design of new materials and in the development of drugs.
The MO diagram for O2 shows that the molecule has a total of 10 valence electrons. These electrons are filled into the molecular orbitals in the following order: 2s, 2s, 2p, 2p, 2p. The MO diagram can be used to predict the bond order of O2, which is 2. The bond order is a measure of the strength of the bond between two atoms.
FAQs on MO Diagram for O2
Here are some frequently asked questions about the molecular orbital (MO) diagram for O2, along with their answers:
Question 1: What is an MO diagram?
Answer: An MO diagram is a graphical representation of the molecular orbitals of a molecule. It shows the energy levels of the orbitals and the number of electrons in each orbital.
Question 2: How can I use an MO diagram to predict the chemical bonding of a molecule?
Answer: The MO diagram can be used to predict the bond order of a molecule, which is a measure of the strength of the bond between two atoms.
Question 3: What is the bond order of O2?
Answer: The MO diagram for O2 shows that the molecule has a bond order of 2, which indicates a strong covalent bond between the two oxygen atoms.
Question 4: How can I use an MO diagram to understand the reactivity of a molecule?
Answer: The MO diagram can provide insights into the reactivity of a molecule by showing the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). A smaller HOMO-LUMO gap indicates a higher reactivity.
Question 5: What are the magnetic properties of O2?
Answer: The MO diagram for O2 shows that the molecule has two unpaired electrons, which makes it paramagnetic.
Question 6: How can I use an MO diagram to design new materials?
Answer: MO diagrams can be used to design new materials by understanding the electronic structure and properties of molecules. This information can be used to tailor the properties of materials for specific applications.
Summary: MO diagrams are powerful tools for understanding the electronic structure, bonding, and properties of molecules. They can be used to predict a variety of properties, including bond order, reactivity, and magnetic properties.
Transition: For more information on MO diagrams, please refer to the following resources:
Conclusion on MO Diagram for O2
In this article, we have explored the molecular orbital (MO) diagram for O2. We have learned that the MO diagram is a powerful tool for understanding the electronic structure, bonding, and properties of molecules. The MO diagram for O2 shows that the molecule has a total of 10 valence electrons, which are filled into the molecular orbitals in the following order: 2s, 2s, 2p, 2p, 2p. The MO diagram can be used to predict the bond order of O2, which is 2. The bond order is a measure of the strength of the bond between two atoms.
We have also learned that the MO diagram can be used to understand the reactivity, magnetic properties, and other properties of molecules. MO diagrams are a valuable tool for chemists and other scientists who are interested in understanding the electronic structure and properties of molecules.
Youtube Video: