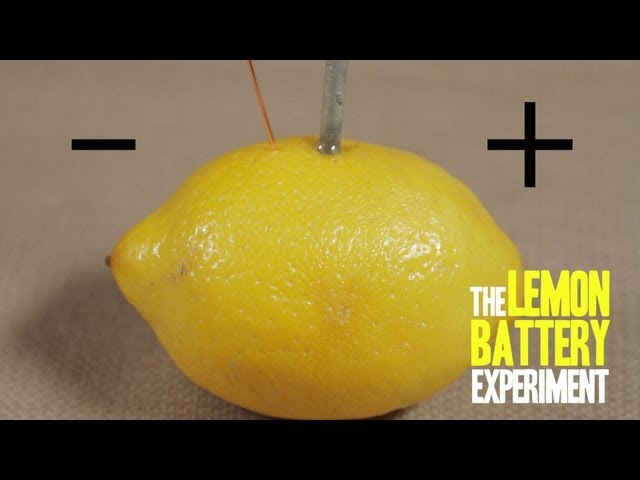
A lemon battery science experiment is a simple and fun way to demonstrate how a battery works. It is a great way to teach children about the basics of electricity and chemistry.
To make a lemon battery, you will need:
- A lemon
- Two pieces of metal (such as copper and zinc)
- A voltmeter
To make the battery, insert the two pieces of metal into the lemon, making sure that they do not touch each other. Connect the voltmeter to the two pieces of metal. You should see the voltmeter reading a voltage of around 1 volt.
The lemon battery works because the lemon contains citric acid, which is an electrolyte. The electrolyte allows the ions in the metal to flow from one piece of metal to the other, creating an electrical current.
Lemon batteries are not very powerful, but they can be used to power small devices, such as LEDs or calculators. They are also a great way to teach children about the basics of electricity and chemistry.
The lemon battery science experiment is a fun and educational way to learn about the basics of electricity and chemistry. It is a great activity for children of all ages.
Lemon Battery Science Experiment
A lemon battery science experiment is a valuable educational tool that demonstrates fundamental electrical principles. Here are eight key aspects to consider:
- Electrochemical Reaction: Oxidation-reduction reaction between metals and acid.
- Electrical Circuit: Closed loop for electron flow, including lemon electrolyte.
- Voltage Generation: Chemical energy converted to electrical energy, measured in volts.
- Current Flow: Electrons move through the circuit, creating a current measured in amps.
- Simple Construction: Easily assembled with accessible materials like lemons, metals, and a voltmeter.
- Educational Value: Illustrates concepts of electrochemistry, energy conversion, and circuit analysis.
- Alternative Energy Source: Demonstrates potential for sustainable and renewable energy.
- Fun and Engaging: Hands-on activity that sparks interest in science and technology.
These aspects highlight the lemon battery experiment’s value in understanding basic electrical principles. It showcases the conversion of chemical energy into electrical energy and the role of electrochemical reactions in generating voltage and current. The simplicity and low cost of the experiment make it an excellent educational tool, fostering interest in science and alternative energy sources.
Electrochemical Reaction
In the lemon battery science experiment, the electrochemical reaction between metals and acid is crucial for generating electrical energy. This reaction involves the transfer of electrons between the metal electrodes and the acid electrolyte (lemon juice). Here’s a detailed look at the process:
- Oxidation at the anode: Zinc (Zn) atoms lose electrons and undergo oxidation, forming positively charged zinc ions (Zn2+) that dissolve in the electrolyte.
- Reduction at the cathode: Copper (Cu) ions in the electrolyte gain electrons and undergo reduction, forming neutral copper atoms (Cu) that deposit on the copper electrode.
- Electron flow: The electrons released by zinc atoms flow through the external circuit, creating an electrical current.
- Ionic movement: To maintain electrical neutrality, positively charged zinc ions move towards the cathode, while negatively charged chloride ions (Cl–) from the electrolyte move towards the anode.
This electrochemical reaction establishes a continuous flow of electrons and ions, generating a potential difference (voltage) between the electrodes and sustaining the electrical current in the lemon battery.
Electrical Circuit
In a lemon battery science experiment, establishing a closed electrical circuit is essential for the generation and flow of electricity. This circuit provides a complete pathway for electrons to travel, enabling the electrochemical reaction to take place and sustain an electrical current.
- Components of the Circuit: The lemon battery circuit consists of the lemon (electrolyte), two metal electrodes (anode and cathode), and an external wire connecting the electrodes. The lemon acts as the electrolyte, facilitating the movement of ions between the electrodes.
- Electron Flow: When the circuit is complete, electrons released by the oxidation of zinc atoms at the anode flow through the external wire towards the copper cathode. This electron flow creates an electrical current in the circuit.
- Ionic Movement: To maintain electrical neutrality, positively charged zinc ions migrate from the anode to the cathode through the electrolyte, while negatively charged chloride ions move in the opposite direction.
- Significance in Lemon Battery Experiment: The closed electrical circuit is crucial for the lemon battery experiment as it allows for the continuous flow of electrons and ions, generating a potential difference (voltage) and sustaining the electrical current.
Understanding the role of the electrical circuit in the lemon battery experiment provides insights into the fundamental principles of electrochemistry and the conversion of chemical energy into electrical energy.
Voltage Generation
In the lemon battery science experiment, voltage generation is a crucial aspect that demonstrates the conversion of chemical energy into electrical energy. Voltage, measured in volts, represents the electrical potential difference between the two electrodes (anode and cathode) of the lemon battery.
The electrochemical reaction between zinc and copper in the presence of lemon juice (electrolyte) generates a voltage due to the transfer of electrons. Zinc atoms undergo oxidation, releasing electrons that flow through the external circuit towards the copper electrode, where they reduce copper ions. This electron flow creates an electrical current and establishes a voltage difference between the electrodes.
The magnitude of the voltage generated in the lemon battery experiment depends on several factors, including the concentration of the electrolyte (lemon juice), the surface area of the electrodes, and the distance between them. Higher concentrations, larger surface areas, and shorter distances generally lead to higher voltage generation.
Understanding voltage generation in the lemon battery experiment provides insights into the fundamental principles of electrochemistry and the conversion of chemical energy into electrical energy. It also highlights the importance of voltage as a measure of electrical potential and its practical applications in various electronic devices and systems.
Current Flow
In the context of a lemon battery science experiment, current flow is a fundamental aspect that demonstrates the movement of electrons through the circuit and the generation of an electrical current. Current, measured in amperes (amps), is the rate of flow of electric charge through a conductor or circuit.
- Electron Movement: In the lemon battery experiment, electrons released by the oxidation of zinc atoms at the anode travel through the external wire towards the copper cathode. This movement of electrons constitutes the electric current in the circuit.
- Circuit Completeness: For current to flow, a complete circuit is necessary. In the lemon battery, the lemon electrolyte, metal electrodes, and connecting wire form a closed loop that allows electrons to circulate.
- Measurement and Significance: The magnitude of the current generated in the lemon battery can be measured using an ammeter. A higher current indicates a greater flow of electrons and a stronger electrical current.
- Factors Affecting Current: The strength of the current in the lemon battery experiment is influenced by factors such as the concentration of the electrolyte, the surface area of the electrodes, and the resistance of the circuit.
Understanding current flow in the lemon battery science experiment provides insights into the principles of electrochemistry, the conversion of chemical energy into electrical energy, and the role of current in various electrical applications.
Simple Construction
The simplicity of the lemon battery science experiment lies in the ease with which it can be constructed using readily available materials. Lemons, metals such as copper and zinc, and a voltmeter are commonly found items that can be easily obtained, making the experiment accessible to a wide range of individuals.
This simple construction is a key aspect of the lemon battery science experiment, as it allows for easy replication and exploration of fundamental electrical principles. The use of everyday materials enables students, hobbyists, and enthusiasts to conduct the experiment without the need for specialized equipment or complex setups.
The simplicity of the lemon battery science experiment serves as a valuable educational tool, fostering hands-on learning and encouraging experimentation. By constructing and testing the lemon battery, individuals can gain practical insights into the generation of electrical energy through electrochemical reactions and the basic principles of electrical circuits.
Educational Value
The lemon battery science experiment holds immense educational value as it serves as a practical demonstration of fundamental electrochemical principles, energy conversion, and circuit analysis. Through hands-on exploration, this experiment provides a tangible understanding of abstract concepts, making them more accessible and relatable.
The electrochemical reaction between zinc and copper in the presence of lemon juice showcases the conversion of chemical energy into electrical energy. This process illustrates the principles of electrochemistry, including oxidation-reduction reactions and the generation of voltage. By measuring the voltage and current output of the lemon battery, students can explore the relationship between chemical reactions and electrical energy production.
Furthermore, the lemon battery experiment demonstrates the basic components of an electrical circuit, including the power source (lemon battery), conducting wires, and a load (voltmeter). By manipulating these components, students can investigate the effects of different circuit configurations on current flow and voltage. This hands-on approach fosters an understanding of circuit analysis and the behavior of electrical circuits.
In summary, the lemon battery science experiment serves as an effective educational tool for illustrating the concepts of electrochemistry, energy conversion, and circuit analysis. It provides a practical and engaging platform for students to explore these fundamental principles, fostering a deeper understanding of the underlying mechanisms that power our technological world.
Alternative Energy Source
The lemon battery science experiment offers a tangible example of an alternative energy source, showcasing the potential for sustainable and renewable energy generation. By harnessing the electrochemical reaction between zinc and copper in the presence of lemon juice, the experiment demonstrates the conversion of chemical energy into electrical energy without relying on fossil fuels.
- Renewable Nature: Unlike fossil fuels, lemons are a renewable resource that can be grown and replenished naturally, making the lemon battery a sustainable energy source.
- No Greenhouse Gas Emissions: The electrochemical process in the lemon battery does not produce greenhouse gases, contributing to the fight against climate change and promoting a cleaner environment.
- Educational Value: The lemon battery experiment serves as an educational tool to raise awareness about alternative energy sources and their potential benefits.
- Limitations and Future Prospects: While the lemon battery may not be a practical large-scale energy source due to its limited power output, it highlights the possibilities for exploring other organic and renewable materials for efficient energy generation.
Overall, the lemon battery science experiment provides a glimpse into the potential of alternative energy sources, emphasizing the importance of sustainable and renewable energy solutions for a cleaner and more sustainable future.
Fun and Engaging
The lemon battery science experiment stands out as a captivating and engaging hands-on activity that effectively ignites curiosity and fosters an interest in science and technology among individuals of all ages.
- Interactive Learning Experience: The experiment involves active participation and manipulation of materials, allowing learners to engage with scientific concepts in a tactile and memorable way.
- Visual and Sensory Stimulation: The experiment produces visible results, such as the generation of electricity, which stimulates the senses and enhances comprehension.
- Real-World Applications: By demonstrating the principles of electrochemistry and energy conversion, the experiment connects science to everyday life, making it more relatable and meaningful.
- Problem-Solving and Critical Thinking: The experiment encourages learners to ask questions, troubleshoot, and develop critical thinking skills as they explore the factors that affect the lemon battery’s performance.
The lemon battery science experiment aligns perfectly with the goals of sparking interest in science and technology. It provides a fun and engaging platform for learners to discover fundamental scientific principles, develop their problem-solving abilities, and cultivate a lifelong passion for scientific inquiry.
Frequently Asked Questions about Lemon Battery Science Experiment
This section addresses common concerns and misconceptions surrounding the lemon battery science experiment, providing concise and informative answers.
Question 1: What is the purpose of a lemon battery science experiment?
A lemon battery science experiment demonstrates the principles of electrochemistry and energy conversion. It shows how a simple combination of lemon juice, metals, and a voltmeter can generate electricity.
Question 2: How does a lemon battery work?
A lemon battery works through an electrochemical reaction between zinc and copper in the presence of lemon juice. The lemon juice acts as an electrolyte, allowing ions to flow and create an electrical current.
Question 3: What materials are needed for a lemon battery?
To construct a lemon battery, you will need a lemon, two metal electrodes (such as copper and zinc), a voltmeter, and connecting wires.
Question 4: How much electricity can a lemon battery produce?
The amount of electricity produced by a lemon battery is relatively small. It can generate around 1 volt of electricity, which is enough to power small devices like LEDs or calculators.
Question 5: Is a lemon battery a sustainable energy source?
While a lemon battery is not a practical large-scale energy source, it demonstrates the potential for alternative energy generation. It highlights the possibility of using renewable materials for sustainable energy production.
Question 6: What are the educational benefits of a lemon battery science experiment?
The lemon battery science experiment is a valuable educational tool. It teaches fundamental concepts of electrochemistry, energy conversion, and circuit analysis in a fun and engaging way.
Summary: The lemon battery science experiment is a simple yet effective way to demonstrate the principles of electrochemistry and energy conversion. It is a versatile tool for education, showcasing the potential of alternative energy sources and sparking interest in science and technology.
Transition to the next article section: This concludes the frequently asked questions about the lemon battery science experiment. Continue reading to explore advanced applications and variations of this experiment.
Tips for Conducting a Lemon Battery Science Experiment
The lemon battery science experiment is a valuable educational tool for demonstrating principles of electrochemistry and energy conversion. Here are some tips to ensure successful and informative experimentation:
Tip 1: Choose Fresh Lemons
Fresh lemons provide a more acidic electrolyte, resulting in a stronger electrochemical reaction and higher voltage output. Avoid using old or spoiled lemons.
Tip 2: Use Clean Electrodes
Clean metal electrodes (copper and zinc) ensure good electrical contact and minimize resistance. Sandpaper or a wire brush can be used to remove any oxidation or impurities.
Tip 3: Insert Electrodes Deeply
Inserting the electrodes deeply into the lemon ensures maximum surface area contact with the electrolyte, leading to increased current flow and voltage generation.
Tip 4: Connect the Voltmeter Correctly
Connect the positive terminal of the voltmeter to the copper electrode (cathode) and the negative terminal to the zinc electrode (anode). Incorrect connections will result in incorrect voltage readings.
Tip 5: Measure Voltage and Current
Use a voltmeter to measure the voltage generated by the lemon battery. Additionally, a multimeter can be used to measure the current flowing through the circuit.
Tip 6: Explore Variations
Experiment with different types of citrus fruits (oranges, limes) or other acidic liquids (vinegar, soda) to observe how they affect the battery’s performance.
Tip 7: Educational Applications
Use the lemon battery experiment to teach concepts of electrochemistry, energy conversion, and circuit analysis in science classrooms or educational settings.
Summary: By following these tips, you can conduct successful lemon battery science experiments that effectively demonstrate fundamental scientific principles and promote a deeper understanding of electrochemistry and energy conversion.
Transition to the article’s conclusion: These tips provide a comprehensive guide for conducting informative and engaging lemon battery science experiments. With careful execution and exploration, this experiment can serve as a valuable educational tool and spark interest in the fascinating world of electrochemistry.
Conclusion
The lemon battery science experiment serves as a simple yet powerful tool for exploring fundamental principles of electrochemistry and energy conversion. Through hands-on experimentation, individuals can gain practical insights into the generation of electricity from chemical reactions and the behavior of electrical circuits.
This article has comprehensively explored the lemon battery science experiment, discussing its educational value, importance as an alternative energy source, and fun and engaging nature. By providing clear explanations, practical tips, and addressing frequently asked questions, this article aims to empower readers with the knowledge and tools to conduct successful and informative lemon battery experiments.
The lemon battery science experiment continues to be a valuable educational resource, fostering interest in science and technology among students of all ages. Its versatility and simplicity make it an excellent platform for demonstrating abstract scientific concepts in a tangible and engaging way.
As we continue to explore alternative energy sources and promote scientific literacy, the lemon battery science experiment will undoubtedly remain a relevant and impactful tool. Its ability to spark curiosity, ignite passion, and provide a foundation for further scientific exploration is a testament to its enduring significance.
Youtube Video: